KinetiLeaseTM
KinetiSolTM Equipment Leasing Program
Harness the power of KinetiSol Technology in your laboratory
Now You Can Access KinetiSolTM Within Your Own Lab
KinetiLease is a practical and flexible alternative to traditional outsourcing for companies that wish to integrate this advanced technology into their in-house development laboratories.
AustinPx’s KinetiSol Technology is an award winning manufacturing solution that enables the rapid creation of amorphous solid dispersions (ASD) without the use of solvents, enhancing the bioavailability of poorly soluble drug molecules, including molecules with high melting points and poor solvent solubility. This results in higher exposure, faster processing times, and a more sustainable approach to drug development. For over a decade, KinetiSol Technology has delivered superior ASD performance, scalability, and sustainability, benefiting global pharmaceutical and biotech companies alike.
Through AustinPx's fee-for-service CDMO service offering, the KinetiSol Technology has been utilized by global pharmaceutical and biotech companies and applied to more than 60 molecules.
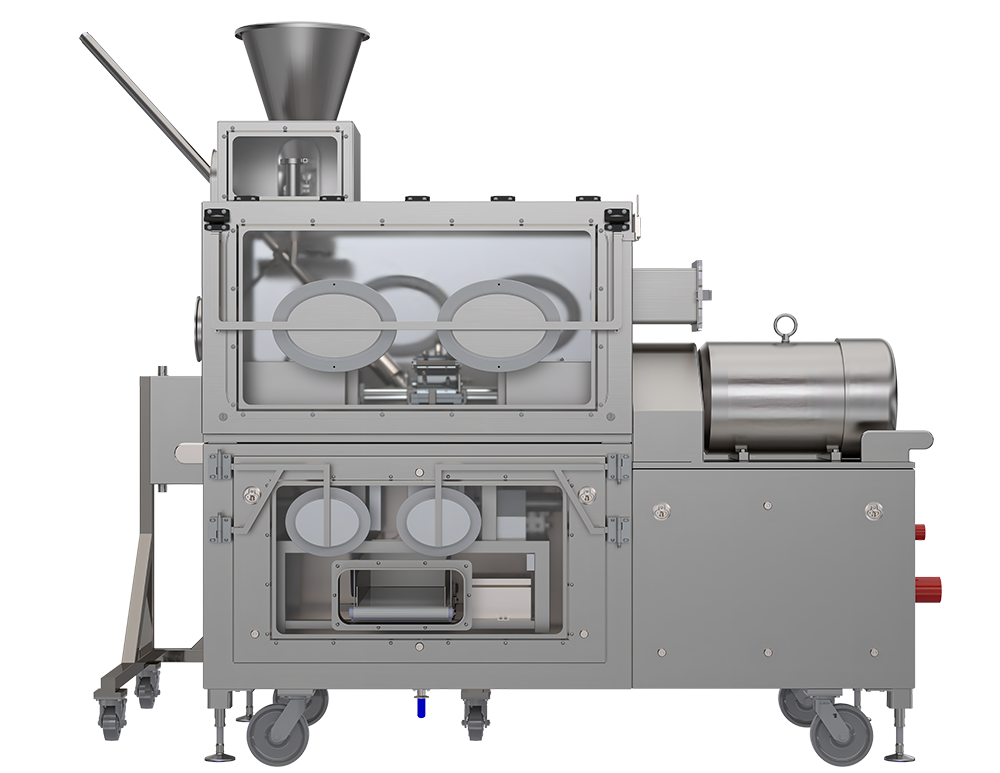
With its ease of use and small operational footprint, KinetiSol provides a faster, simpler, and more efficient means of producing ASD formulations compared to other ASD technologies. Throught the KinetiLease program, companies can install KinetiSol's research-scale equipment - the KinetiSol Formulator - within their in-house laboratories, while having comprehensive technical support from AustinPx.
See how the KinetiSol Formulator Works

KinetiSol Formulator Technical Specifications
Processing Capacity:
7 to 25 grams / shot
Up to 200 grams / hour
Electrical:
480 Volt, 60 Hertz, 3-Phase, Alternating
30 Amp Time-Delayed fuse or circuit breaker
Weight:
Approximately 1,500 lbs.
Dimensions:
47” x 70” x 53” (L x H x D)
Containment:
Non-hazardous, OEL 2 (100 – 1000 µg/m3)
Additional containment can be purchased
Network:
Cat5 network connection
Benefits of the KinetiSol Technology
KinetiSol’s unique and highly optimized equipment design offers many advantages over other amorphous dispersion technologies including:
- Wider Formulation Design Space: The KinetiSol system opens the ASD space to more APIs, including those with high melting points and poor solvent solubility, as well as a broader range of excipients, including thermally labile, highly viscous, non-thermoplastic and innovative mixtures.
- Superior ASD Performance: KinetiSol Solid Dispersions have been shown to provide increased exposure, improved compressibility, higher ASD loading and favorable downstream processing characteristics, leading to reduced pill burden.
- Faster Development: Rapid processing and change over for faster throughput of prototypes and exploration of a broader formulation design space.
- Scalability: The equipment is designed to easily scale from the R&D to the commercial scale equipment for faster timelines and streamlined development.
- Sustainability: With its non-solvent process and significantly smaller footprint, KinetiSol is a greener and more sustainable ASD technology.
- Extended product life cycle: Possible IP creation through patented processing technology and composition of matter patents.

Key Aspects of the KinetiLease Program
- Comprehensive Training: AustinPx offers extensive technical training to ensure the smooth onboarding and adoption of the technology.
- Technical Support: An AustinPx Technical Support Specialist will be assigned to your KinetiLease project to ensure optimal training and utilization throughout the life of the Lease.
- White Glove Delivery & Installation: To ensure that the KinetiSol Formulator equipment is properly installed, an AustinPx representative will deliver and be onsite for installation, commissioning and calibration of the the KinetiSol Formulator equipment according to the specifications.
- Annual Preventative Maintenance
Formulation Development and Manufacturing with KinetiSol Technology
AustinPx offers comprehensive development, analytical testing and GMP manufacturing support for KinetiSol Technology enhanced APIs at all stages of development, including the handling of highly potent molecules. KinetiSol Technology has been developed at research and production scales to ensure speed to clinic and easy scale up to commercial production. Click here for more details regarding our CDMO services for KinetiSol.
From Bench to Commercial
From Bench to Commercialization, KinetiSol's high-throughput, continuous processing and small footprint translates to a lower operational cost.
KinetiSol R&D Scale Equipment
Batch Sizes: 5-20g | Rate: 5-200 g/hr
KinetiSol Pilot-to-Commercial Scale Equipment
Continuous Processing | Rate: Up to 20kg/hr*
Contact Us
We invite you to test the performance of your candidate using our proprietary KinetiSol Technology. Contact Us to Learn More about KinetiSol Technology.